On June 26, 2024, Lecanemab, a highly anticipated new drug for targeted treatment of Alzheimer's disease, was prescribed for the first time in China. This progress marks a new stage in the field of precision medicine for AD in China.
So what do you need to know about this new drug?
What is Leqembi (Lecanemab)?
Lecanemab, Chinese name for lenkanizumab, is A human anti-Aβ antibody.
It can bind to β-amyloid oligomers, which are abnormally accumulated in the brain of patients with Alzheimer's disease, promote the clearance of β-amyloid in the brain, reduce the further accumulation of plaques, thereby changing the pathology of the disease and alleviating the progression of the disease.
On July 6, 2023, the Food and Drug Administration (FDA) announced full approval of Leqembi (Lecanemab). It is also the first new Alzheimer's drug to receive full FDA approval in 20 years.
Why is the launch of this new drug causing such a stir?
Unlike the drugs we are familiar with now, such as Aricept and memantine, which are "symptomatic treatments", Lecanemab, like neck lymphaticovenous anastomosis, a surgical treatment for AD initiated by professor Xie Qingping of Hangzhou Qiushi Hospital, directly acts on the clearance of β-amyloid in the brain. Simply put, it is to solve the problem from the "root", that is, "treatment of the cause".

In any case, we are well on the way to cure the cause.

Is Lecanemab Effective?
The emergence of new drugs is welcome, but there is really only one thing that everyone is concerned about:
Does it work?
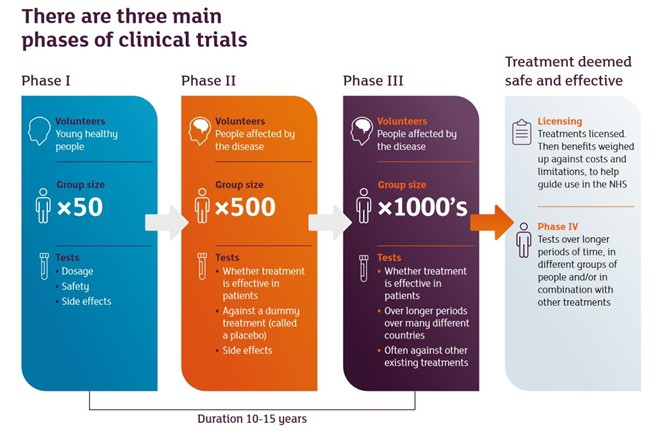
Lecanemab was tested in a trial called CLARITY AD, a "phase 3" trial - usually the final stage of drug testing.
During the trial, about 900 patients with early Alzheimer's disease received lecanemab for 18 months, while another group of 900 received a dummy drug (placebo).
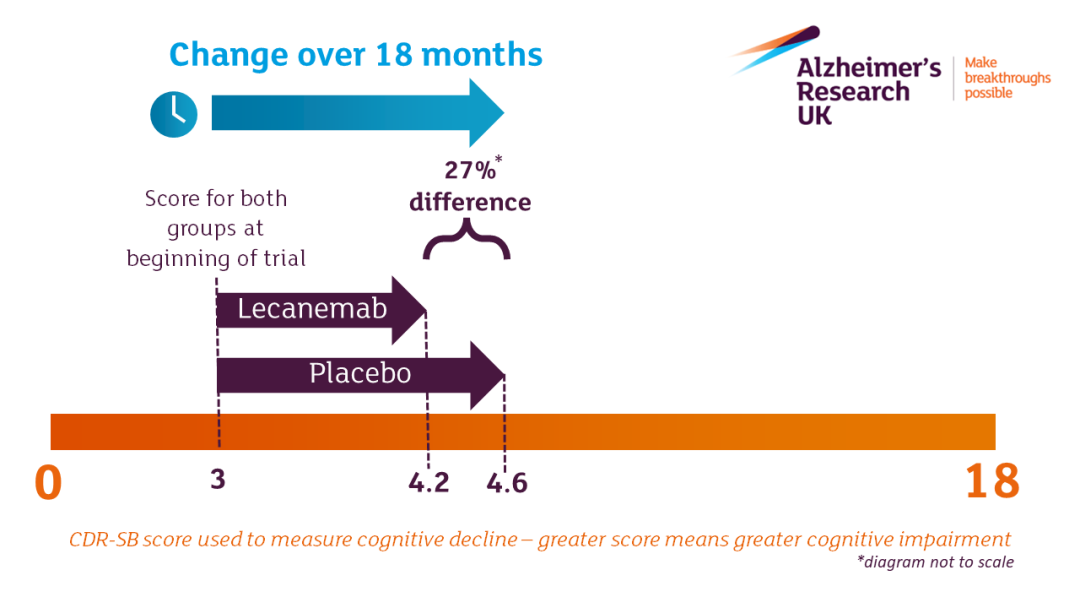
The CLARITY AD results show that:
A statistically significant difference between Lecanemab and placebo was seen at 6 months of treatment.
After 18 months of treatment, the rate of cognitive and memory decline was 27% slower with Lecanemab.
In addition, Lecanemab significantly reduced brain amyloid load and delayed tau deposition. Based on model predictions, Leqembi delayed progression to moderate Alzheimer's disease by 2-3 years in patients with early Alzheimer's disease.
Lecanemab, from the results of the experiment, is effective.
More studies are needed to confirm this, of course, as the trial has so far only followed subjects for 18 months.

Disadvantages and side effects of Lecanemab
Now that Lecanemab has been shown to be effective, who should use it? What are the side effects of this drug?
First, Lecanemab, as a monoclonal antibody, is not indicated for prophylactic long-term treatment.
It is important to emphasize that Lecanemab only slows cognitive decline, it does not reverse it, and it does not cure Alzheimer's disease.
Second, Lecanemab is currently indicated only in patients in the early stages of Alzheimer's disease.
Lecanemab, when used early in Alzheimer's disease, did slow disease progression, allowing "more time to participate in daily life and to live independently.
However, Lecanemab has not been shown to be effective in patients with moderate-to-severe Alzheimer's disease and is therefore not approved for the treatment of such patients.
Then there is the question of the side effects of Lecanemab.
Risks associated with Lecanemab therapy include brain swelling and cerebral hemorrhage.
Brain swelling:
In clinical studies, brain swelling was found in nearly 13% of patients treated with lecanemab, as compared with approximately 2% of untreated patients.
Most of these cases are mild to moderate, do not cause obvious symptoms, and the brain swelling symptoms usually resolve within 4 months.
However, about 3% of patients will experience severe side effects of brain swelling, including headache, visual impairment and confusion.

Intracerebral hemorrhage:
Cerebral hemorrhage occurred in approximately 17% of patients treated with lecanemab versus 9% of untreated patients, and the most common symptom associated with hemorrhage was dizziness.
Cerebral hemorrhage is the most worrisome side effect, and although a large cerebral hemorrhage is rare, every precaution should be taken to minimize the risk of cerebral hemorrhage.
Lecanemab is also not recommended for:
People who are using anticoagulants;
People with obvious cerebral hemorrhage, brain swelling, aneurysm, vascular malformation or brain tumor;
People with uncontrolled bleeding disorders.
Finally, let's talk about the price of this drug.
Lecanemab was administered intravenously every 2 weeks, with the number of doses administered dependent on the patient's weight.
The recommended dose is: 10 mg/kg body weight. That IS TO SAY, IF THE WEIGHT OF THE PATIENT IS 60 KILOGRAMS, THE MEDICINE THAT NEEDS TO INJECT 600 MILLIGRAMS SO.
If the clinical trial "18 months to complete a treatment cycle" is calculated, the total treatment cost for one and a half years is about 270-300,000 yuan.
That's the general information about the new drug.
A new method of "causal treatment" for AD: deep neck lymphatic-venous anastomosis
In October 2022, the Chinese Journal of Microsurgery (Volume 45, Issue 5, P.570-574) published Professor Xie Qingping's paper "deep cervical lymphatic and venous drainage for the treatment of elderly patients with cognitive impairment under 3D gogle-off". Its principle stems from the discovery in 2015 by American scientists of the mechanism of lymphatic vessels and lymphatic-like circulation system in the brain.
 The deep neck lymphangio-venous anastomosis pioneered by the team of Professor Xie Qingping, president of Hangzhou Qiuzhi Hospital, connects the neck lymphatic vessels directly to the veins for rapid drainage of the neck lymphatic vessels, fully discharges the pathogenic β-amyloid protein and Tau protein in the brain, re-opening the drainage channels of metabolites in the brain, and reduces the lymphatic reflux pressure in the brain. So as to improve the symptoms of brain dysfunction in patients. This surgical procedure has improved the quality of life for many Alzheimer's patients and their caregivers, and has been widely recognized and used for reference by the medical community at home and abroad.
The deep neck lymphangio-venous anastomosis pioneered by the team of Professor Xie Qingping, president of Hangzhou Qiuzhi Hospital, connects the neck lymphatic vessels directly to the veins for rapid drainage of the neck lymphatic vessels, fully discharges the pathogenic β-amyloid protein and Tau protein in the brain, re-opening the drainage channels of metabolites in the brain, and reduces the lymphatic reflux pressure in the brain. So as to improve the symptoms of brain dysfunction in patients. This surgical procedure has improved the quality of life for many Alzheimer's patients and their caregivers, and has been widely recognized and used for reference by the medical community at home and abroad.









